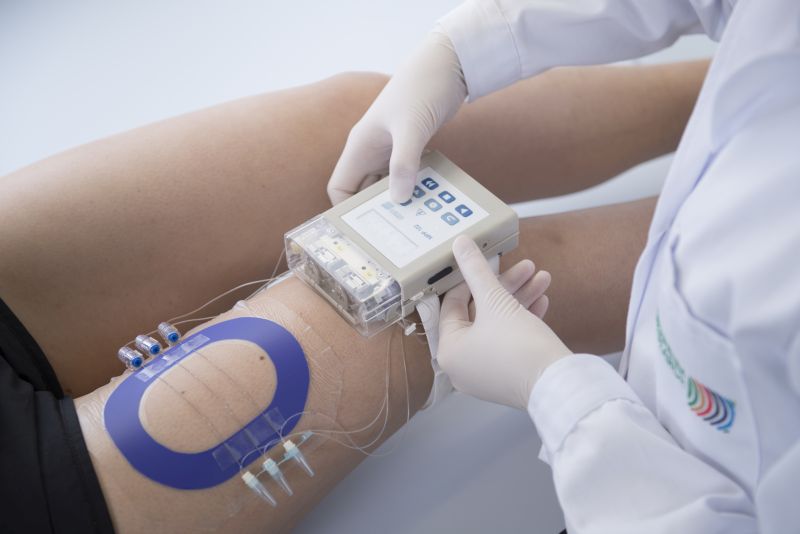
Following the successful completion of the first FDA-cofunded project (dOFM4BE, 2013-2015), the Institute HEALTH has once again won the award for US $ 1,500,000 funding from the US FDA. In the new project, a new cost- and time-saving procedure is being developed to test dermatological generics. Generics are an attractive alternative to the often expensive original products. Currently, however, there are hardly any generic alternatives for topically applied dermatological formulations. The approval of new generics requires the generic product to be bioequivalent to the original preparation which is usually achieved with expensive and time-consuming clinical endpoint studies. The current project "BE-TWO" (Development of a General Bioequivalence Test Method for Topical Drugs using dOFM) aims to develop affordable, standardized methods to assess bioequivalence of dermal drugs by using dermal open flow microperfusion (dOFM). "BE-TWO" will develop a general clinical dOFM setup and a bioequivalence test for lipophilic and protein-bound substances. Ultimately dOFM will be established as a reliable method for accurate, sensitive and reproducible bioequivalence measurements of dermatological products.
![[Translate to English:] [Translate to English:]](/fileadmin/user_upload/FDA_Logo_05.gif)