PK/PD Studies
PK/PD in Skin
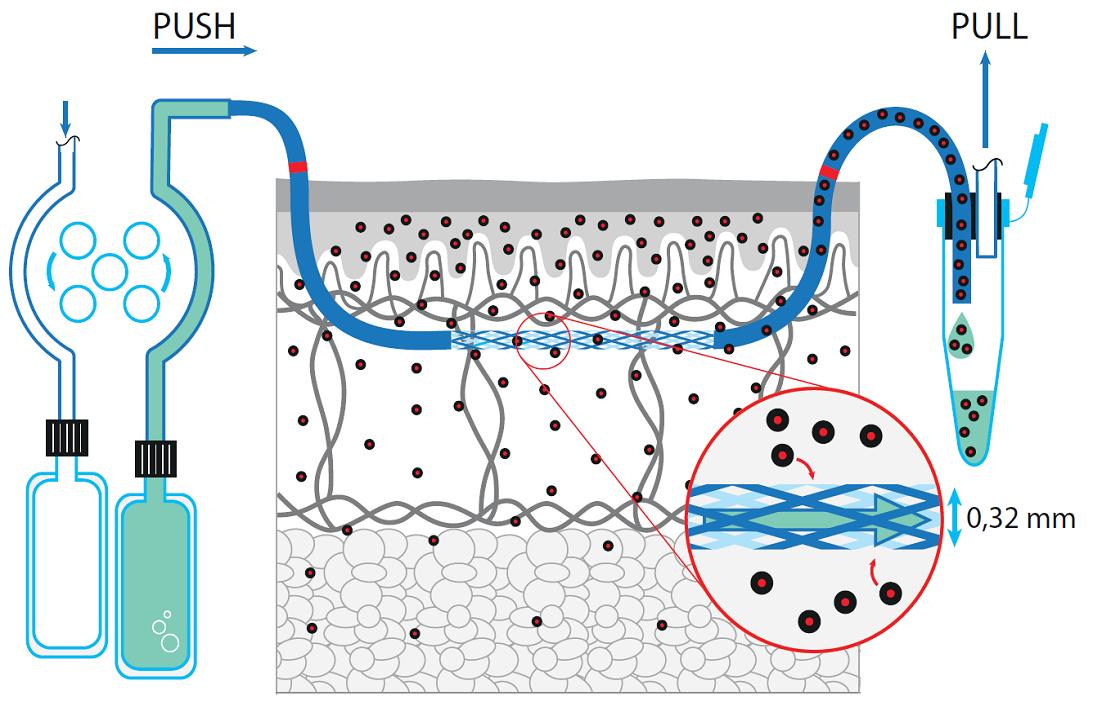
We provide you with information about the pharmacokinetics (PK) and pharmacodynamics (PD) of a particular component directly at an active site in skin using our dermal OFM technology. Dermal OFM can provide valuable data very early in the preclinical and clinical phase of drug development.
Your Benefits
- PK and PD of systemically or topically administered drugs can be measured continuously for more than 48 hours after their administration.
- Even at an early stage of drug development, we can identify whether a dermatologically-active ingredient penetrates the outermost layer of the epidermis (stratum corneum) and reaches the target tissue (dermis).
- New formulations are tested in low-dose experiments by applying the dermatological agent to very small areas. Thus, we reduce the risk to the patient.
- PK data can be collected much more rapidly from skin during the process of drug development than in phase 1 clinical trials. Highly relevant PK/PD data are available about two years earlier than would be possible following normal drug development procedures.
- By performing several measurements simultaneously on lesion and non- lesion sites in a study subject, we can reduce between-subject variability and increase statistical power. We can reduce the number of test subjects required by gathering enough data to determine drug effectiveness during the clinical Phase 1 trial. Thus, we lower the risk of redesigning a drug after a clinical Phase 3 trial, after hundreds of study participants have already been tested.
- The costs and development time can be kept to a minimum because we can determine the pharmacological profile of a drug at an early stage of development. Pharmaceutical companies can learn quickly whether a substance is a potential drug candidate.
Materials and Methods
Study materials
- CE-marked probes, pumps and accessories for preclinical and clinical studies
- Minimally invasive, membrane-free probes with a 0.36 x 15 mm exchange area and a 0.5 mm needle
- Portable pump (0.1-10 ul/min) with Delta push-pull modes
- 3 OFM probes per pump
- Patents for the probes and pump are pending
Complementary methods
- Ultrasound to verify the probe location and measure dermal thickness
- Tape stripping technique for proteomic analysis
- Skin biopsies, only for validation purposes
- Suction blister technique, only for validation purposes