- About us
- Research fields
- DIGITAL – Institut für Digitale Technologien
- MATERIALS – Institut für Sensorik, Photonik und Fertigungstechnologien
- ROBOTICS – Institut für Robotik und Flexible Produktion
- COREMED – Zentrum für Regenerative Medizin und Präzisionsmedizin
- HEALTH – Institut für Biomedizinische Forschung und Technologien
- LIFE – Institut für Klima, Energiesysteme und Gesellschaft
- POLICIES – Institut für Wirtschafts-, Sozial und Innovationsforschung
- Business areas
- Products & Services
- Research infrastructure
- Beteiligungen
- Career
- Aktuelles
- Publications
- Kontakt zu uns
Lipidosa
PROJECT DURATION:
07/2025
—
06/2027
Achaeosoma
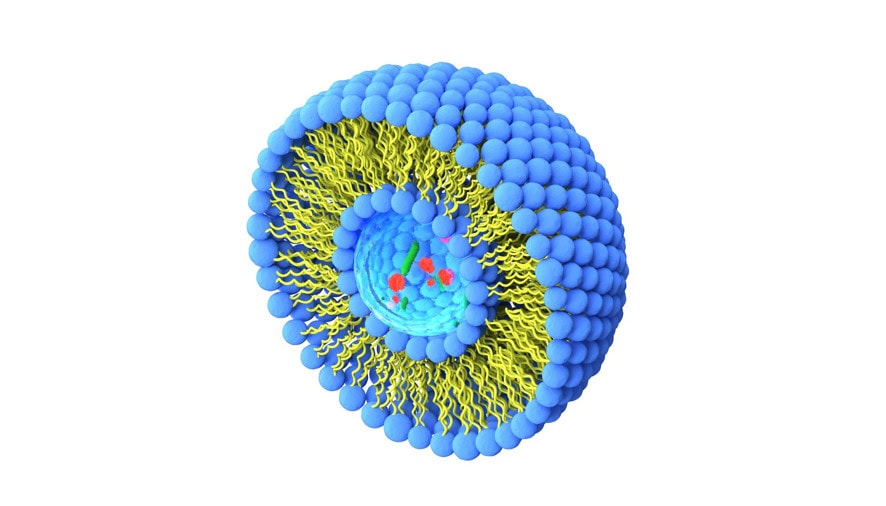
The Project
A key challenge in drug development is delivering active ingredients to their target site at effective concentrations. Oral administration is simple and preferred, but many drugs are unstable in the digestive tract and poorly absorbed. Encapsulating them in stable liposomes made of archaelipids—called archaeosomes—may help. However, their behavior in the human body is not well understood and needs further pharmacological investigation.
Our Role in the Project
In this JR will perform the pharmacological characterization of lipid nanoparticles and active ingredients based on highly sensitive bioanalytics and the innovative technology of OFM.
Research groups
Downloads
Customer/Funding Organization
Project Partner
NovoArc
Project Details
One of the greatest challenges in drug development is ensuring that active pharmaceutical ingredients (APIs) reach their target in the body—in the right place, at the right dosage, and at the right time. Of the various routes of administration, oral administration remains the simplest, most practical, and most preferred method for patients. However, numerous promising active ingredients face problems with this method of administration: they can be broken down in the aggressive environment of the digestive tract or fail to effectively cross the intestinal barrier, resulting in poor bioavailability.
To overcome these limitations, researchers have explored encapsulating drugs in liposomes—tiny spherical vesicles that can protect and transport active compounds. A particularly promising type of liposome is the archaeosome, which is made from archaelipids (specifically, tetraether lipids, or TEL). Archaeosomes are remarkably stable, especially under acidic and enzymatic conditions, making them ideal candidates for oral drug delivery.
Despite their potential, the use of archaeosomes in humans is still limited. This is largely due to a lack of comprehensive data on how they interact with the human body after ingestion. Critical aspects such as their stability in the bloodstream, breakdown in the liver, ability to cross the intestinal barrier, and overall distribution in organs have not yet been systematically studied.
The LIPIDOSA project is designed to fill this knowledge gap by conducting the first systematic pharmacological evaluation of archaeosomes as drug carriers for oral administration. At the core of this study is octreotide, a peptide-based drug that will be encapsulated in archaeosomes to assess their effectiveness as a delivery system.
Key areas of investigation include:
In vitro: Assessing the stability of archaeosomes in blood and their metabolism in the liver.
Ex vivo: Examining how the encapsulation of octreotide—and variations in archaeosome composition—affect absorption across the intestinal mucosa.
In vivo: Studying the impact of archaeosome-encapsulated drugs on pharmacokinetics—specifically, the absorption, distribution, metabolism, and excretion (ADME) of both the drug and the carrier lipids in the body.
Towards Regulatory Readiness and Clinical Use: By generating detailed data on how archaeosomes behave in the human body, LIPIDOSA lays the foundation for their future clinical use. These insights can serve as essential scientific and regulatory prerequisites for the approval of archaeosomes as a safe and effective platform for oral drug delivery.
With LIPIDOSA, we aim to move one step closer to more efficient, patient-friendly therapies—by making oral delivery a viable option even for complex and sensitive drug compounds.
Project team
Related Projects
Miteinander zukunftsrelevant
Die JOANNEUM RESEARCH ist Innovations- und Technologieanbieter im Bereich der angewandten Forschung. Als Forschungsgesellschaft der Länder und Regionen prägen wir mit unseren Forschungskompetenzen die Entwicklung unserer modernen Gesellschaft und Wirtschaft nachhaltig und menschenzentriert. Als multidisziplinäres Team in flexiblen, innovationsfreundlichen Strukturen leben wir höchste gesellschaftliche und wissenschaftliche Ansprüche.







