- About us
- Research expertise
- DIGITAL – Institut für Digitale Technologien
- MATERIALS – Institut für Sensorik, Photonik und Fertigungstechnologien
- ROBOTICS – Institut für Robotik und Flexible Produktion
- COREMED – Zentrum für Regenerative Medizin und Präzisionsmedizin
- HEALTH – Institut für Biomedizinische Forschung und Technologien
- LIFE – Institut für Klima, Energiesysteme und Gesellschaft
- POLICIES – Institut für Wirtschafts-, Sozial und Innovationsforschung
- Business areas
- Products & Services
- Research infrastructure
- Beteiligungen
- Career
- Aktuelles
- Publications
- Kontakt zu uns
Infrastructure for Pharmacological Research
Instruments and methods for the measurement of pharmacokinetics and pharmacodynamics in different tissues.
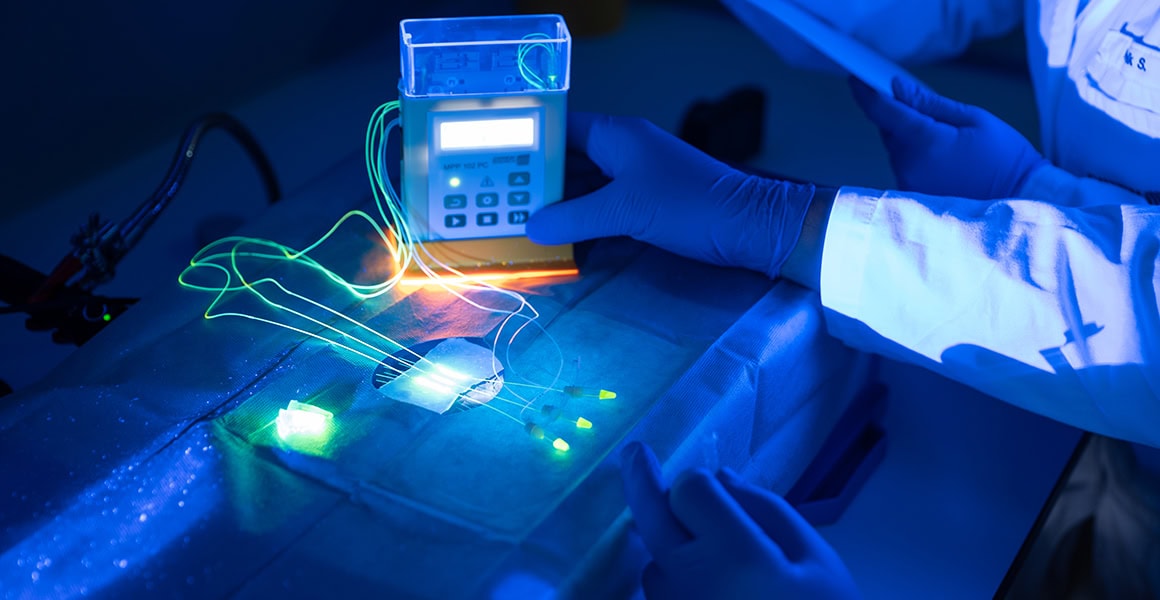
Open Flow Microperfusion (OFM) - Technology
OFM enables the continuous collection of interstitial fluid in (pre)clinical studies for drug development and biomarker research.
OFM is a patented, probe-based and membrane-free method for the continuous collection of minute amounts of interstitial fluid from various tissues. It enables access to the entire biochemical information of the interstitium and thus opens up completely new perspectives in preclinical and clinical (pharmacological) research and drug testing. The membrane-free OFM probes have an exchange surface without a diffusion barrier, so that sampling is not restricted by the size, lipophilicity of substances or protein binding effects.
- Applications: pharmacokinetics, pharmacodynamics, bioequivalence, bioavailability, dose finding, drug metabolism
- Substances: active pharmaceutical ingredients (low molecular weight to antibody-drug conjugates; fat- or water-soluble), endogenous substances (substances ranging from small molecules to cells)
- Preclinical models: pig, rat, mouse
- Tissue: skin, mucous membrane, brain, muscle, fatty tissue, lymph, cancer
Preclinical Research
We manufacture OFM materials for preclinical studies and distribute them worldwide.
- Products: information on the various OFM products, our product catalog, application videos, FAQs or the instructions for use can be found here
- Sales regions and countries: Europe, North, Central and South America, Japan, China, South Korea, Hong Kong, Singapore, Australia, Russia, Israel, India. Write us and we will put you in touch with your local sales partner.
Clinical Research
- OFM materials for clinical studies are approved as medical devices and CE certified.
- Applications: skin, muscle, fatty tissue
Pharmacological Studies
- In Vitro Release Tests (IVRT): testing the release of active ingredients from creams, ointments, and gels (semi-solid dosage formulations) with Franz diffusion cells Learn more
- Ex-Vivo Tests: transdermal or transepidermal transport of active ingredients from creams, ointments and gels with human skin explants, artificial or cultured skin, with continuous or point measurement methods (open microperfusion, microdialysis, biopsies, tape stripping) Learn more
- Preclinical Studies: preclinical in-vivo evaluation of PK/PD profiles in healthy and diseased animal models (mouse, rat, pig) in different tissues - skin, brain, adipose tissue, muscle, mucosa, lymph
- Clinical Studies: clinical investigation of PK/PD profiles in healthy volunteers and patients directly in the target tissue - skin, adipose tissue
- Bioanalysis: detection of biological or pharmaceutical substances in biological samples from clinical and preclinical studies
- Metabolomics: Specific (targeted) and broad (untargeted) metabolomics analyses for the determination and quantification of metabolites from metabolic processes for biomarker research
Explore Services
- Pharmacokinetics, Pharmacodynamics, Bioequivalence
- Bioanalysis, Pharmaceutical Analysis and Metabolomics

Your contact
Standort
Das sagen unsere Kunden
More Research Infrastructure
Bioanalyses for pharacokinetic/phamacodynamic studies, metabolomics for clinical research and pharmaceutical analyses
Fully validated online system for the electronic recording of patient data in clinical trials (eCRFs).
State-of-the-art laboratory infrastructure for (bio)analytical, pharmacological and medical technology studies.
Miteinander zukunftsrelevant
Die JOANNEUM RESEARCH ist Innovations- und Technologieanbieter im Bereich der angewandten Forschung. Als Forschungsgesellschaft der Länder und Regionen prägen wir mit unseren Forschungskompetenzen die Entwicklung unserer modernen Gesellschaft und Wirtschaft nachhaltig und menschenzentriert. Als multidisziplinäres Team in flexiblen, innovationsfreundlichen Strukturen leben wir höchste gesellschaftliche und wissenschaftliche Ansprüche.



