Pharmacokinetics, Pharmacodynamics, Bioequivalence
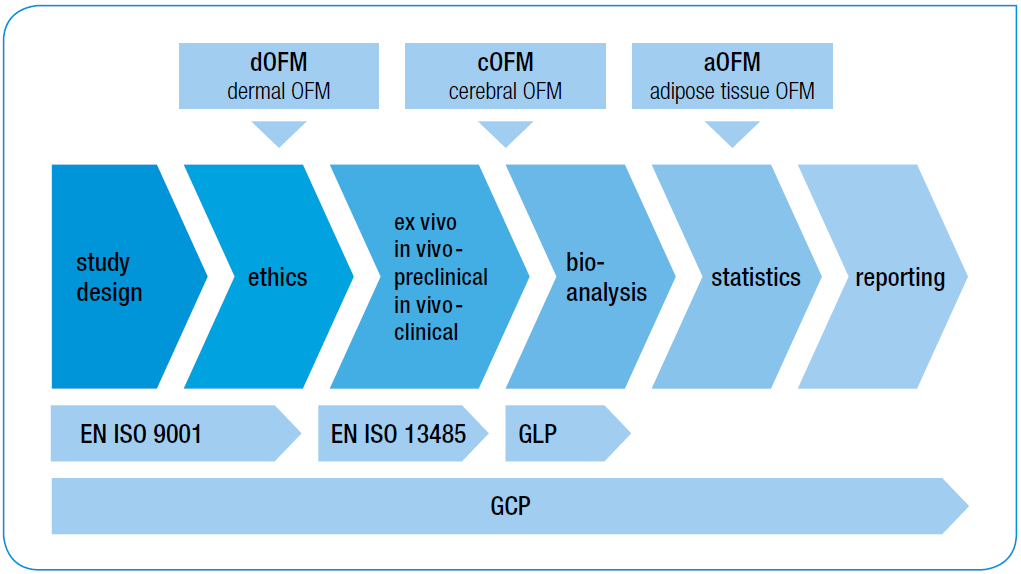
As a service to the pharmaceutical industry, we investigate the pharmacokinetics (PK) and pharmacodynamics (PD) of new drugs as well as the bioequivalence (BE) of new pharmaceutical formulations. We also carry out biomarker-based and metabolomic studies to pinpoint the causes of inflammatory diseases. We perform preclinical ex vivo and in vivo, as well as clinical in vivo, studies. During these studies, we use microdialysis and our patented Open Flow Microperfusion (OFM).
We focus on the targeted tissue
Dermatology: Transport routes and effects of drugs within the dermal layer after topical or systemic administration (e.g., topical glucocorticoids or systemic antibodies in the case of psoriasis) [Details]
Endocrinology: Transport routes and effects of drugs in adipose tissue (e.g., insulin) [Details]
Neurology: Transport across the blood-brain barrier (BBB), dose-response effects when the BBB is bypassed, monitoring of BBB function (e.g., highly lipophilic substances, psycho- and neuropharmaceuticals [Details]
Open-flow microperfusion
- OFM is a method whereby tissue fluids are continuously extracted, which uses minimally invasive, membrane-free probes.
- It allows direct access to the interstitial spaces and fluid.
- Interstitial fluid can be continuously collected in vivo while several parameters (including biomarkers) are simultaneously analyzed, without any restrictions in terms of size (to the cellular level), lipophilicity of measured compounds, or protein binding effects.
- OFM allows access to all biochemical information within the interstital space in vivo and, thus, opens new perspectives for preclinical and clinical drug testing.
- Learn more about OFM technology